Name:2,4,6-Trichloro-1,3,5-triazine
CA Name: 1,3,5-Triazine,2,4,6-trichloro-
Molecular Structure:
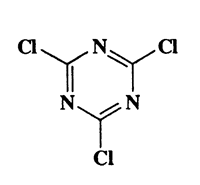
184.41,2,3,4,5-triazine,6-trichloro-,6-Trichloro-1,C3Cl3N3,CAS 108-77-0
Molecular Formula:C3Cl3N3
Molecular Weight: 184.41
CAS Registry Number:108-77-0
List of synthetic dyes :
C.I.Disperse Yellow 92
C.I.Vat Orange 18
C.I.Reactive Orange 1
C.I.Reactive Orange 113
C.I.Reactive Orange 12
C.I.Reactive Orange 122
C.I.Reactive Orange 124
C.I.Reactive Orange 13
C.I.Reactive Orange 14
C.I.Reactive Orange 2
C.I.Reactive Orange 20
C.I.Reactive Orange 35
C.I.Reactive Orange 38
C.I.Reactive Orange 4
C.I.Reactive Orange 5
C.I.Reactive Orange 82
C.I.Reactive Orange 84
C.I.Reactive Black 1
C.I.Reactive Black 39
C.I.Reactive Black 42
C.I.Reactive Black 45
C.I.Reactive Black 8
C.I.Reactive Red 1
C.I.Reactive Red 108
C.I.Reactive Red 11
C.I.Reactive Red 12
C.I.Reactive Red 120
C.I.Reactive Red 125
C.I.Reactive Red 13
C.I.Reactive Red 141
C.I.Reactive Red 15
C.I.Reactive Red 189
C.I.Reactive Red 194
C.I.Reactive Red 195
C.I.Reactive Red 2
C.I.Reactive Red 221
C.I.Reactive Red 222
C.I.Reactive Red 223
C.I.Reactive Red 224
C.I.Reactive Red 225
C.I.Reactive Red 227
C.I.Reactive Red 231
C.I.Reactive Red 239
C.I.Reactive Red 24
C.I. Reactive Red 24:1
C.I.Reactive Red 240
C.I.Reactive Red 241
C.I.Reactive Red 250
C.I.Reactive Red 252
C.I.Reactive Red 253
C.I.Reactive Red 256
C.I.Reactive Red 261
C.I.Reactive Red 29
C.I.Reactive Red 3
C.I.Reactive Red 31
C.I.Reactive Red 33
C.I.Reactive Red 4
C.I.Reactive Red 43
C.I.Reactive Red 45
C.I.Reactive Red 45:1
C.I.Reactive Red 6
C.I.Reactive Red 7
C.I.Reactive Red 79
C.I.Reactive Red 8
C.I.Reactive Red 88
C.I.Reactive Red 9
C.I.Reactive Red 92
C.I.Reactive Yellow 1
C.I.Reactive Yellow 105
C.I.Reactive Yellow 135
C.I.Reactive Yellow 145
C.I.Reactive Yellow 160
C.I.Reactive Yellow 167
C.I.Reactive Yellow 176
C.I.Reactive Yellow 178
C.I.Reactive Yellow 179
C.I.Reactive Yellow 18
C.I.Reactive Yellow 185
C.I.Reactive Yellow 193
C.I.Reactive Yellow 2
C.I.Reactive Yellow 202
C.I.Reactive Yellow 3
C.I.Reactive Yellow 35
C.I.Reactive Yellow 4
C.I.Reactive Yellow 5
C.I.Reactive Yellow 81
C.I.Reactive Yellow 84
C.I.Reactive Yellow 86
C.I.Reactive Blue 1
C.I.Reactive Blue 109
C.I.Reactive Blue 13
C.I.Reactive Blue 14
C.I.Reactive Blue 15
C.I.Reactive Blue 160
C.I.Reactive Blue 171
C.I.Reactive Blue 194
C.I.Reactive Blue 198
C.I.Reactive Blue 2
C.I.Reactive Blue 214
C.I.Reactive Blue 216
C.I.Reactive Blue 221
C.I.Reactive Blue 222
C.I.Reactive Blue 231
C.I.Reactive Blue 255
C.I.Reactive Blue 256
C.I.Reactive Blue 4
C.I.Reactive Blue 40
C.I.Reactive Blue 49
C.I.Reactive Blue 5
C.I.Reactive Blue 63
C.I.Reactive Blue 7
C.I.Reactive Blue 74
C.I.Reactive Blue 81
C.I.Reactive Green 19
C.I.Reactive Green 6
C.I.Reactive Green 8
C.I.Reactive Violet 1
C.I.Reactive Violet 2
C.I.Reactive Violet 46
C.I.Reactive Violet 8
C.I.Reactive Brown 1
C.I.Reactive Brown 10
C.I.Reactive Brown 44
C.I.Reactive Brown 7
C.I.Reactive Brown 8
C.I.Reactive Brown 9
C.I.Basic Red 111
C.I.Acid Yellow 127
C.I.Acid Yellow 158
C.I.Acid Yellow 158:1
C.I.Fluorescent Brightener 1
C.I.Fluorescent Brightener 103
C.I.Fluorescent Brightener 104
C.I.Fluorescent Brightener 114
C.I.Fluorescent Brightener 117
C.I.Fluorescent Brightener 145
C.I.Fluorescent Brightener 17
C.I.Fluorescent Brightener 204
C.I.Fluorescent Brightener 205
C.I.Fluorescent Brightener 208
C.I.Fluorescent Brightener 210
C.I.Fluorescent Brightener 220
C.I.Fluorescent Brightener 225
C.I.Fluorescent Brightener 234
C.I.Fluorescent Brightener 24
C.I.Fluorescent Brightener 243
C.I.Fluorescent Brightener 245
C.I.Fluorescent Brightener 251
C.I.Fluorescent Brightener 260
C.I.Fluorescent Brightener 264
C.I.Fluorescent Brightener 28
C.I.Fluorescent Brightener 31
C.I.Fluorescent Brightener 32
C.I.Fluorescent Brightener 353
C.I.Fluorescent Brightener 357
C.I.Fluorescent Brightener 362
C.I.Fluorescent Brightener 49
C.I.Fluorescent Brightener 71
C.I.Fluorescent Brightener 79
C.I.Fluorescent Brightener 83
C.I.Fluorescent Brightener 85
C.I.Fluorescent Brightener 87
C.I.Fluorescent Brightener 9
C.I.Fluorescent Brightener 90
C.I.Direct Orange 94
C.I.Direct Red 11
C.I.Direct Red 181
C.I.Direct Red 224
C.I.Direct Red 243
C.I.Direct Red 253
C.I.Direct Red 89
C.I.Direct Yellow 130
C.I.Direct Yellow 138
C.I.Direct Yellow 142
C.I.Direct Yellow 162
C.I.Direct Yellow 83
C.I.Direct Yellow 86
C.I.Direct Green 26
C.I.Direct Green 28
C.I.Direct Green 59


[…] Methods : 2,4,6-Trichloro-1,3,5-triazine with aniline first condensation, then with 2-(2-Hydroxyethylamino)ethanol second condensation, […]
[…] and 2,5-Bichloro-4-(3-methyl-5-oxo-4,5-dihydropyrazol-1-yl)benzenesulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation,the product (2 moles) and then with 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 mol) of […]
[…] acid diazo, and between N-(3-aminophenyl)acetamide coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation chlorine, then with a product between, 2-(3-Aminophenylsulfonyl)ethyl hydrogen sulfate […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 2,4-Diaminobenzenesulfonic acid and aniline condensation, the products of diazo, and […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 4-Hydroxy-7-(methylamino)naphthalene-2-sulfonic acid condensation, its product and the […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 2,4-Diaminobenzenesulfonic acid condensation, condensation products diazo, and […]
[…] in alkaline conditions and 7-Amino-4-hydroxynaphthalene-2-sulfonic acid coupling, and then and 2,4,6-Trichloro-1,3,5-triazine and 3-Aminobenzenesulfonic acid condensation, eventually forming copper […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcondensation, 2-Aminobenzenesulfonic acid […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 7-Amino-4-hydroxynaphthalene-2-sulfonic acid condensation, 2-Amino-5-methoxybenzenesulfonic […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine in turn and 7-Amino-4-hydroxynaphthalene-2-sulfonic acid and ethyl […]
[…] conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, its products and 2,4,6-Trichloro-1,3,5-triazine condensation , and then and the 4-Aminobenzenesulfonic […]
[…] alkaline conditions and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcoupling, its products and 2,4,6-Trichloro-1,3,5-triazine and 2-Amino-4-sulfobenzoic […]
[…] acid and 2,4-Diaminobenzenesulfonic acid condensation, again with 2,4,6-Trichloro-1,3,5-triazine condensation […]
[…] acid diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acidcoupling, and then and 2,4,6-Trichloro-1,3,5-triazine condensation […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine respectively with chlorine 2,4-Diaminobenzenesulfonic acid and ethyl […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and N-(3-aminophenyl)acetamide, condensation, 7-Aminonaphthalene-1,3,6-trisulfonic acid […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 3-Aminobenzenesulfonic acid and 2,5-Diaminobenzenesulfonic acid condensation, Its product (2 […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine with 2-Aminobenzene-1,4-disulfonic acid and N-(3-aminophenyl)acetamide between condensation, […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine and 2 Moore’s C.I. Mordant Yellow 12 (C.I. 14045) and 1 Moore’s neighbors […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine first and 3-Amino-4-hydroxybenzenesulfonamide diazo, and 7-Amino-4-hydroxynaphthalene-2-sulfonic […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine in turn and (1) 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, (2) 4-Nitrobenzenamine diazo, and […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine in turn and (1) 1-Amino-4-(4-aminophenylamino)-9,10-dioxo-9,10-dihydroanthracene-2-sulfonic acid, […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine in turn and (1) 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid, (2) 4-Nitrobenzenamine diazo, and […]
[…] Methods : 2-Aminobenzene-1,4-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine first condensation, and 2,4-Diaminobenzenesulfonic acid second condensation, its product diazo, and […]
[…] and 2,4,6-Trichloro-1,3,5-triazine first condensation, and 2,4-Diaminobenzenesulfonic acid second condensation, and then diazotization […]
[…] Methods : 2,4-Diaminobenzenesulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation chlorine, product diazo, and 1-(3-Sulfophenyl)-3-methyl-5-pyrazolone coupling, An […]
[…] product diazo, and 8-Aminonaphthalene-1-sulfonic acid coupling, again with 2,4,6-Trichloro-1,3,5-triazine condensation, then […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, 2-Aminobenzenesulfonic acid diazo, coupled with condensation products, and then and […]
[…] acid diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, and 2,4,6-Trichloro-1,3,5-triazine condensation, again with 2-Amino-5-sulfobenzoic acid […]
[…] Methods : 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid and 2,4,6-Trichloro-1,3,5-triazine condensation, aniline diazo, and condensation products coupled, then […]
[…] hydrogen sulfate diazo, and 4-Amino-5-hydroxynaphthalene-2,7-disulfonic acid coupling, again with 2,4,6-Trichloro-1,3,5-triazine condensation, the last and the 3-Aminobenzenesulfonic acid […]
[…] and 5-Aminonaphthalene-2-sulfonic acid coupling, product diazo, Coupled with m-Toluidine ,and 2,4,6-Trichloro-1,3,5-triazine condensation, again with 2-Aminobenzene-1,4-disulfonic acid […]
[…] Methods : first use 2,4,6-Trichloro-1,3,5-triazine(2 Moore) deal with 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore), then and ammonia reaction, its […]
[…] Methods :first will be 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore) and 2,4,6-Trichloro-1,3,5-triazine (2 Moore) condensation, then the products and aniline (2 Moore) and 2-(2-Hydroxyethylamino)ethanol […]
[…] Methods : 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore) and 2,4,6-Trichloro-1,3,5-triazine (2 Moore) condensation, then the products and 3-Aminobenzenesulfonic acid (2 Moore) and […]
[…] Methods : 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore), 2,4,6-Trichloro-1,3,5-triazine (2 Moore) and aniline (4 Moore) with […]
[…] Methods : 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore), 2,4,6-Trichloro-1,3,5-triazine (2 Moore) and 2-(2-Hydroxyethylamino)ethanol (4 Moore) with […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine (2 Moore) and 1,2-Bis(4-amino-2-sulfophenyl)ethene (1 Moore) first condensation, then in 30 ℃ and […]
[…] Methods :at 0 ℃ will 2,4,6-Trichloro-1,3,5-triazine (2 Moore) acetone solution and sodium carbonate solution at the same time slowly add people to […]
[…] Methods : 2,4,6-Trichloro-1,3,5-triazine(1 Moore) and 2,4,6-Trichloro-1,3,5-triazine (2 Moore) condensation, again with 2-Aminoethanol(2 Moore) […]